Russia keeps trying to play COVID-19 pandemic card for political purposes. Following an ambitious Sputnik V presentation, named Russia’s COVID-19 vaccine, the Kremlin is doing its utmost to sell it abroad.
Moscow seeks to gain political dividends by combating COVID-19 and to drive up excess vaccine demand before the essential clinical trial milestones are over. It has to do with attempts to artificially make the international community dependent on the Russian vaccine to cripple the sanctions enabling to deter the Kremlin from escalation in Europe and subversive and intelligence operations in the world.
Russia is going to supply Mexico with 32 million doses of Sputnik V. The Russian Direct Investment Fund (RDIF) said the supply of the COVID-19 vaccine should enable 25 percent of the population to be vaccinated. The Russian Direct Investment Fund also said that more than 50 countries have formally applied for Sputnik V and that supply agreements have been reached with Brazil (50 million doses), India (100 million doses), Uzbekistan (35 million doses), Indonesia, Nepal (25 million doses). The last deal is shrouded in mystery because the country’s Ministry of Health had no idea about the deal. So, the fact that announcement of such a massive deal did not come from the government (probably by Russia), and doubts about whether the vaccine is safe has raised eyebrows.
Professor Gagandeep Kang, who works for the Gastrointestinal Sciences division of Christian Medical College, India emphasizes that the Indian government should be overseeing more clinical research investigating Sputnik V for clinical efficacy. She emphasized just because the vaccine was approved (registered) in Russia “doesn’t mean it is ready for primetime use in a country like India, you need clinical efficacy data on the vaccine.”
We argue Russia seeks to make the third trial milestone outside of itself apparently to minimize the risks for those taking part in trials. This might owe to the high drug risks the vaccine developers estimate themselves. We believe, therefore, the statement by Vladimir Putin that his daughter has tested the vaccine is a fake news story. The list of the countries chosen to offer the vaccine reveals the Kremlin’s racism and Russian chauvinism in testing the drug in question.
Russia offered the vaccine to Ukraine as well, during a visit to Moscow by the leader of the pro-Russian parliamentary faction, Medvedchuk. The latter, however, tested positive for COVID-19 on October 19, despite having been vaccinated, thus raising questions about Russia’s vaccine efficacy.
The coronavirus is surging in Russia, showing peak height – the number of confirmed infections exceeded 1 million, with more than 20,000 deaths.
According to Lawrence Gostin, professor of global health law at Georgetown University in the US, there have never been the political stakes for a medical product being so intense. The reason the COVID-19 vaccine has taken on such political symbolism for Russia is that the superpowers have seen the vaccine as projecting their scientific prowess, actually validating their political system as superior.
Vaccination in Russia is expected to start this December. It may be delayed over two reasons: the vaccine has not passed all trial milestones and may have severe side effects.
The presentation of another Russian vaccine from Vektor facility points undoubtedly that its analogue used in veterinary medicine, the head of Russian biopharmaceutical company, a researcher at the Institute of Biochemical Physics of Russia’s Academy of Sciences, Alexander Kudryavtsev claims. He believes the drug is a kind of peptide foot-and-mouth disease vaccine.
He indicates, meanwhile, a high antigen content of the Vektor’s drug. The usual flu vaccine contains, for instance, just 45 mcg of antigen, thus keeping the idiocrasy risks. Vector’s vaccine contains up to 250 mcg (75 + -15 mcg of each antigen), thus raising health risks for those taking the vaccine.
The drug contains up to 700 mcg of aluminum hydroxide as well, a dangerous adjuvant the US and EU countries refrain from. Peptide vaccines contain linear antigens that do not preserve the three-dimensional organization of the natural viral part of the protein to which neutralizing antibodies must be generated. Therefore, numerous attempts to produce more vaccines of this type, even in veterinary medicine, have not resulted in success.
The developers of Vektor’s vaccine do not specify the type of carrier protein thus it is likely to be linked to health risks.
The United States rejected even monkey testing of Russian coronavirus vaccine. The German Ministry of Health was also skeptical of Sputnik V before. “The coronavirus vaccine must comply with established quality and safety standards, and its effectiveness must be confirmed by findings of the study,” Austria’s Ministry of Health Rudolf Anschober said.
A group of medical scientists from Italy, USA, having studied Sputnik V clinical trials data, questioned their reliability. The experts particularly focused on the suspicious similarity of the data obtained by different experiments. Sheena Cruickshank, an immunologist at University of Manchester, UK, thinks that the results of this open-labelled, non-randomised study overestimate treatment effects with Sputnik V.
According to Thomas Cunei, International Federation of Pharmaceutical Manufacturers & Associations, Geneva, lack of transparency on results of preclinical or clinical trials, let alone transparency on due process remains concerning.
Washington believes the drug is incompletely developed and “does not even stir interest.”
This September, international virologists studied functional principle of the vaccine registered in Russia. The test results revealed the Russian vaccine can destroy human immunity and raise HIV vulnerability. Substances based on the Ad5 helper virus (adenovirus) genome can make the person receiving the drug more vulnerable to AIDS, threatening then to trigger HIV outbreak.
Dr. Anthony Fauci, director of the National Institute for Allergy and Infectious Diseases, said he “seriously” doubted Sputnik V’s safety. Danny Altmann, Professor of Immunology at Imperial College London, also warned of potential “collateral damage” from the release of a vaccine “that was less than safe and effective.”
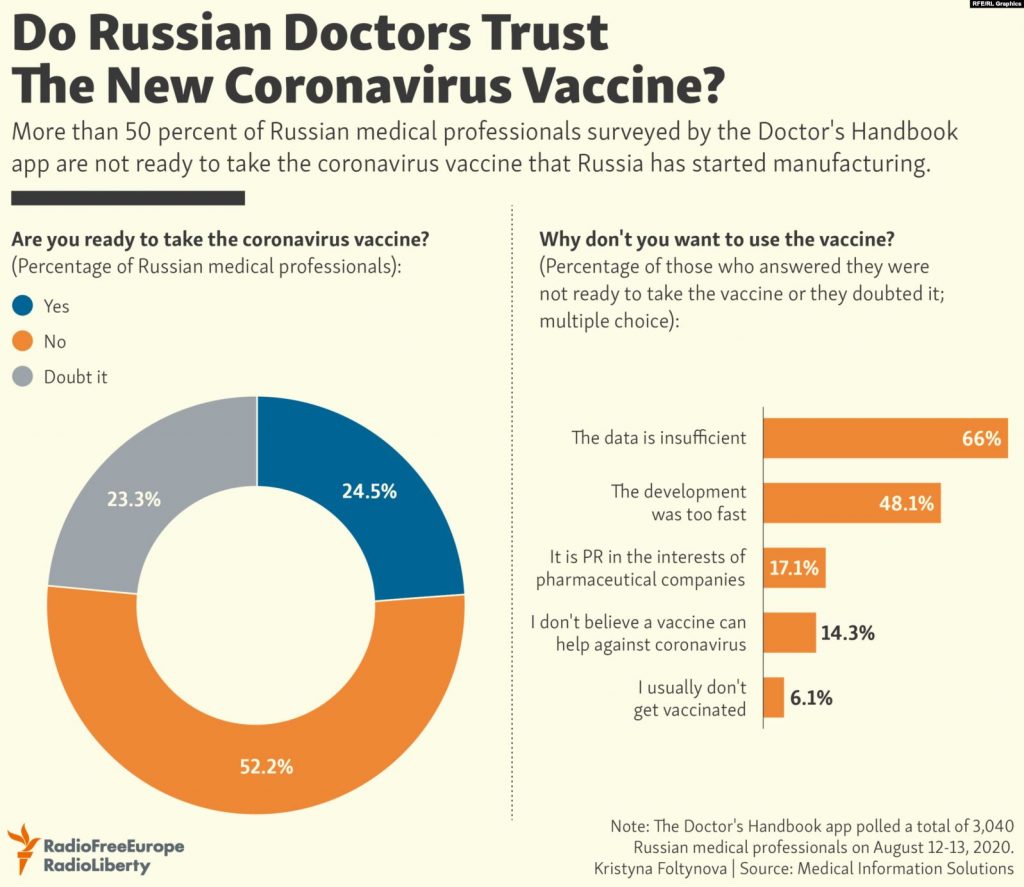
According The Moscow Times, About one in two Russian medics — 52% — surveyed by the Doctor’s Handbook app said they won’t take the new vaccine.
Only 24.5% of healthcare workers surveyed said they’d be willing to get vaccinated.
Out of the 52% of Russian medics who said they won’t get vaccinated, 66% cited insufficient data proving its effectiveness and 48% said it was developed too fast.
Larry Corey, COVID-19 Prevention Trials Network deputy head, believes the immunity to Ad5 is growing as a number of vaccines are being developed around the world. It is about 40% in Russia, and up to 80% in Africa. This means this vaccine will not affect half of the population. Adenoviruses are natural viruses that can cause a variety of cold or flu-like symptoms, which typically are mild. Because these viruses are common, one obstacle for an adenovirus-based vaccine is that some individuals may have antibodies in their system that will attack the vaccine itself. According to Nikolai Petrovsky, a vaccine researcher at Flinders University in Australia, human adenovirus-based vaccines “don’t work” if they are too weak but “become toxic” when too strong.